The cannabis industry is shifting rapidly, and minor cannabinoids are stepping into the spotlight. Among the most discussed are tetrahydrocannabivarin (THCV) and delta-8 tetrahydrocannabinol (delta-8 THC). Both are marketed as uplifting alternatives to delta-9 THC, yet their effects and legal status reveal striking differences. For consumers, the question often becomes whether the “clear-headed” or “gentler” high matches their needs, and for regulators, how to manage compounds that thrive in gray areas of the law.
Delta-8’s “uplifting” profile
Delta-8 THC is a psychoactive isomer of delta-9 that binds CB1 receptors and produces a THC-like high many users describe as gentler. A 500-person academic survey found participants commonly reported relaxation and euphoria with fewer anxiety or paranoia complaints than with traditional cannabis. These findings are self-reported and not placebo-controlled, so conclusions remain provisional, but they help explain consumer enthusiasm.
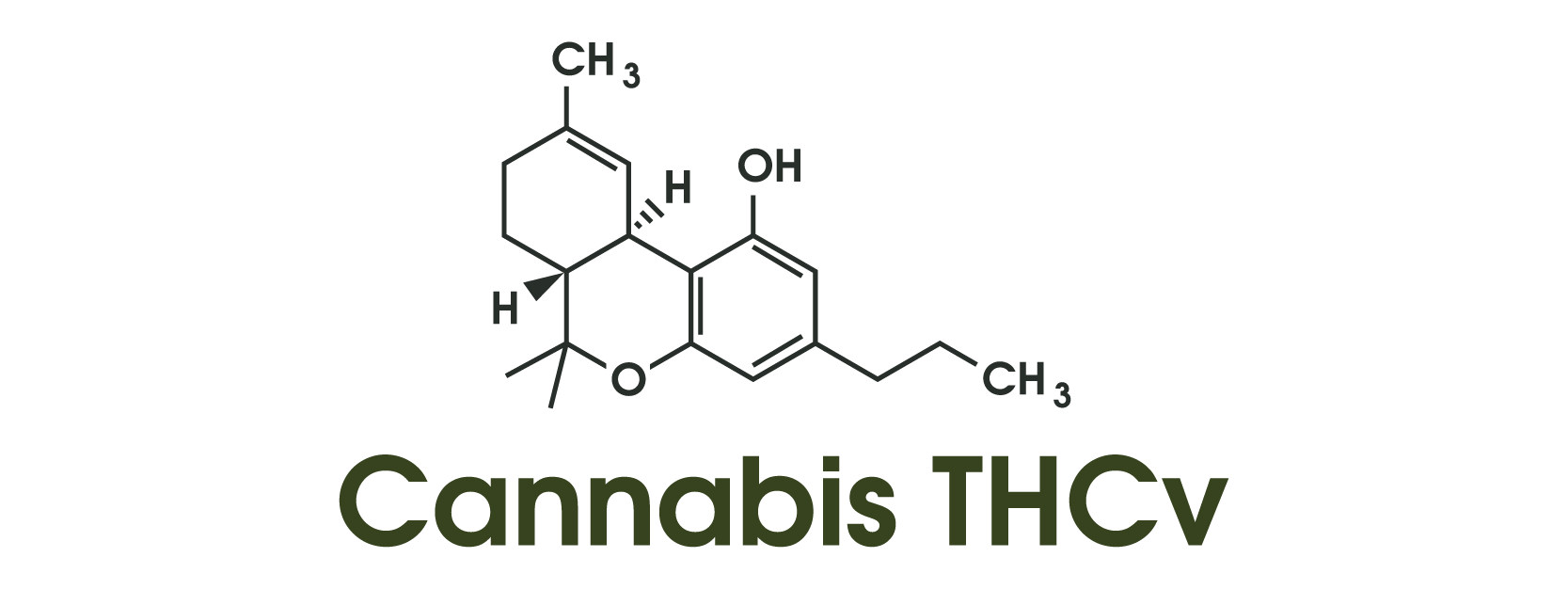
THCV’s “clearer” lift
Tetrahydrocannabivarin (THCV) appears to act primarily as a neutral CB1 antagonist at typical doses, reducing CB1 signaling rather than amplifying it. This activity aligns with reports of a more alert, focused, and “non-stoned” experience. Neuroimaging studies suggest THCV enhances reward-circuit activity without producing classic intoxication, while a small clinical trial in type-2 diabetes patients showed improved glycemic control with no significant psychoactive effects. Early evidence supports the idea that THCV provides a more “clear-headed” effect than THC isomers.
How the “loopholes” emerged
The 2018 Farm Bill excluded hemp from the Controlled Substances Act if delta-9 THC levels remained at or below 0.3% by dry weight. This opened space for psychoactive hemp-derived products so long as delta-9 remained under the threshold. In 2022, the Ninth Circuit’s AK Futures decision ruled that hemp-derived delta-8 products can fall within the Farm Bill’s definition, an interpretation widely embraced by the industry. Congressional researchers now refer to this regulatory gap as the “THC loophole.”
Safety and state action
“Legal” status does not equal “safe.” The FDA has not approved delta-8 for any use and warns about variable potency, contaminants, and child-targeted marketing. Between December 2020 and February 2022, the agency received 104 adverse-event reports, while U.S. poison centers logged 2,362 delta-8 exposures. CDC also noted a spike in related calls as products became more available. States responded differently: some banned delta-8, others imposed potency caps, while a few created regulated “low-dose” markets. Congress is now considering whether to redefine hemp by counting all THC isomers, not just delta-9.
Is “synthetic” the line?
Delta-8 exists only in trace amounts naturally, so most commercial products come from isomerized hemp-derived CBD. The DEA maintains that “synthetically derived tetrahydrocannabinols” remain Schedule I substances. In 2023, the agency clarified that lab-created cannabinoids like delta-8-THCO and delta-9-THCO are illegal, even if sourced from hemp. This stance creates direct conflict with the AK Futures ruling, leaving businesses caught between federal, state, and agency positions.
Where THCV fits legally
THCV is not explicitly listed in federal schedules. When extracted from hemp and kept within the 0.3% delta-9 THC limit, it is generally marketed as compliant. However, state laws differ, and proposals to measure “total THC” could affect THCV products that contain or are blended with other intoxicating cannabinoids.
Conclusion
THCV and delta-8 share space in the hemp marketplace but diverge in both effects and regulatory treatment. Delta-8 delivers a mild THC-like high yet faces scrutiny over synthetic production, safety concerns, and uneven state laws. THCV offers a subtler, more focused experience and remains less targeted by regulators, though compliance still hinges on hemp-derived rules. For consumers, the wisest path is to demand lab-tested transparency, stay mindful of state-specific laws, and recognize that “uplifting” does not mean risk-free. For policymakers, these cannabinoids highlight how quickly innovation can outpace legislation, creating challenges that demand nuanced solutions.