Interest in cannabinoids as tools for studying glucose and energy regulation has grown alongside new insights into the endocannabinoid system (ECS). CB1 receptors influence appetite, lipogenesis, hepatic glucose production, and insulin signaling; in animal and human studies, blocking CB1 has been associated with improved insulin sensitivity and metabolic profiles. This background helps frame why researchers are probing non-intoxicating or low-intoxicating cannabinoids for metabolic endpoints—while recognizing that clinical translation remains uncertain.
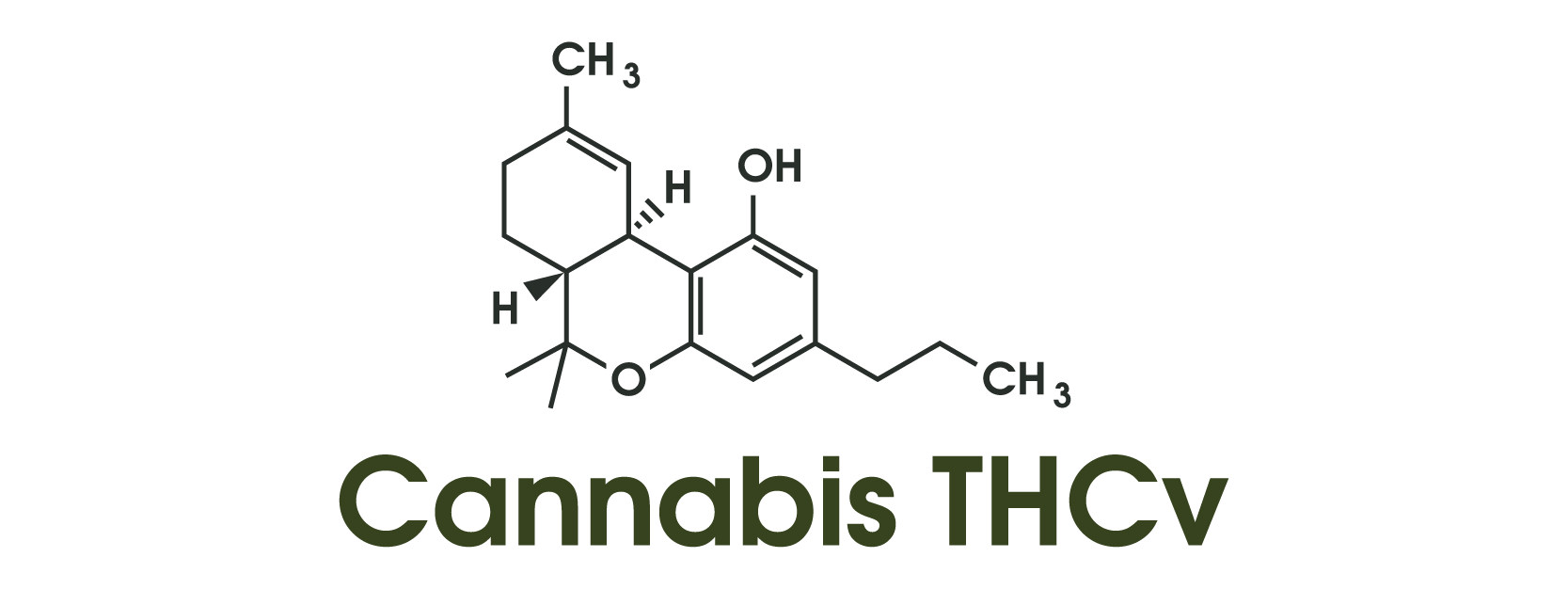
Among phytocannabinoids, tetrahydrocannabivarin (THCV) currently has the most human data related to glycemia. In a randomized, double-blind, placebo-controlled pilot trial of 62 adults with type 2 diabetes not using insulin, participants received THCV (5 mg twice daily), cannabidiol (CBD), CBD/THCV combinations, or placebo for 13 weeks. THCV significantly reduced fasting plasma glucose versus placebo and improved indices of β-cell function; the prespecified primary endpoint (HDL cholesterol) did not change. Combination arms were neutral, and the treatment was generally well tolerated—findings that motivate, but do not prove, therapeutic development. Dose, formulation, and population heterogeneity remain key unknowns for larger trials.
CBD’s picture is different. In the same trial, CBD did not improve glycemic outcomes compared with placebo, though exploratory biomarker shifts (e.g., resistin decrease, GIP increase) were observed versus baseline. Case-level evidence also exists in type 2 diabetes, but single-patient reports cannot establish efficacy. Mechanistically, CBD engages multiple targets (e.g., TRP channels, PPARγ) and may modulate inflammation and stress responses—pathways relevant to metabolic disease—yet clinical effects on blood sugar remain unproven and require adequately powered, controlled studies.
Cannabigerol (CBG) is earlier in its evidence curve. Preclinical work suggests CBG may influence lipid membranes and signaling pathways tied to insulin action. In obese, insulin-resistant rats, CBG exposure was associated with biochemical shifts consistent with improved muscular insulin sensitivity; other basic studies point to CBG-linked changes in sphingolipid metabolism that could intersect with hepatic insulin resistance. These results are hypothesis-generating only: to date, there are no robust human trials assessing CBG on glycemic endpoints.
Mechanistically, THCV is frequently described as a CB1 antagonist/neutral antagonist at lower doses and a partial agonist at CB2, a profile that differs from THC. Interest in CB1 antagonism stems in part from earlier experiences with rimonabant, an effective weight-loss and metabolic drug withdrawn for psychiatric adverse effects; contemporary research explores “neutral” CB1 antagonists that might preserve metabolic benefits with fewer central side effects. THCV’s dose-dependent receptor behavior and peripheral actions remain active areas of inquiry, underscoring the need for careful pharmacology in future trials.
What should researchers prioritize next? First, replicate and extend THCV findings in multi-center, adequately powered trials that track HbA1c, continuous glucose monitoring metrics, body composition, and liver fat over 3–6 months, while standardizing chemovars, dosing, and bioavailability. Second, clarify CBD’s role—if any—in glycemic control by focusing on mechanism-anchored subpopulations (e.g., inflammatory phenotypes) and rigorous placebo comparisons. Third, move CBG into early human pharmacokinetic and safety studies with predefined metabolic endpoints before efficacy testing. Finally, studies should monitor drug–drug interactions and adverse neuropsychiatric signals, given the ECS’s CNS roles.
Bottom line: the ECS is a biologically plausible axis for metabolic research. Early human data nominate THCV as a candidate warranting confirmation, CBD’s effects on glycemia remain inconclusive, and CBG is promising but preclinical. None of these cannabinoids should be viewed as treatments for diabetes at this time; rather, they represent research probes that may, with stronger evidence, inform future therapeutic strategies.